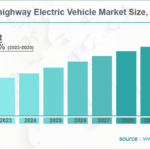
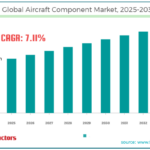
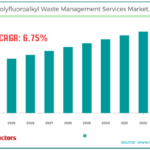
Computational Studies of Diels-Alder Reactions
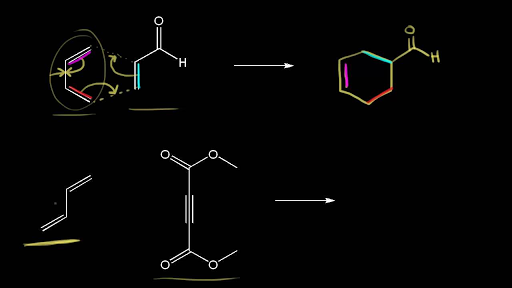
Computational approaches have the potential to make significant contributions to the chemistry and related disciplines. Molecular simulations allow us to learn about chemical reactions at a fraction of the cost of actual experimentation. Thus, it is of the utmost importance to develop solutions that are tailored to the specific issue at hand. They should be as precise as is absolutely essential while also being as quick as possible.
Researchers at the Sorbonne University in Paris, France, including Jean-Philip Piquemal and Riccardo Spezia, looked for inexpensive computational approaches to forecast Diels-Alder reaction features. For a group of “small,” prototypical reactions, they compared the outcomes of density functional theory (DFT) calculations performed using small, “affordable” basis sets (a set of functions used to represent the electronic wave function) with highly accurate, but computationally expensive, approaches. They discovered that despite the slight energy differences between the endo and exo strategies, quick DFT techniques may accurately forecast the diastereoselectivity in Diels-Alder processes involving complex strained cyclic allene dienophiles.
These findings led the researchers to propose a number of DFT functionals that, when combined with a small basis set (6-31G) and dispersion corrections (corrections that account for attractive forces caused by fluctuations in electron density), can provide a good description of Diels-Alder reactions, especially with regard to diastereoselectivity. These reliable, but cost-effective technologies could allow, for example, the simulation of enormous systems.